Sodium Hydroxide Molar Mass
It is prepared by neutralization of phosphoric acid under controlled conditions with sodium hydroxide or sodium carbonate. 418 gL 0 C 1000 gL 25 C 3370 gL 100 C Solubility.
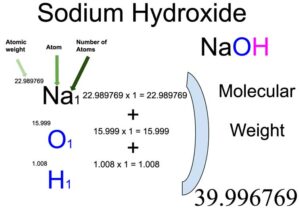
Sodium Hydroxide Naoh Molecular Weight Calculation Laboratory Notes
The pH sensor has been calibrated with pH 7 and pH 10.

. It is a chaotropic salt Uses Food supplement. Request More Information. Once we know the amount of.
Sodium hydroxide solution density table. Sodium hydroxide is an odorless and white crystalline substance that absorbs moisture from the air at the environmental or surrounding temperature. Properties of Sodium Hydroxide NaOH In order to describe its uses it is necessary to describe the features that caustic soda is used in a variety of industries due to these chemical and physical features.
Soluble in glycerol negligible in ammonia insoluble in ether slowly soluble in propylene glycol. Na 3 PO 4. 106498 View Pricing Availability.
Its a synthetic chemical. Molecular Weight Molar Mass. Calculate the precision of your experiment as standard deviation and as deviation.
If your percent deviation is greater than 2 you will need to carry out additional titrations until you have a minimum of three values for the molar. Preparation of Sodium Hydroxide NaOH. Preparation of Sodium Hydroxide.
Sodium iodide chemical formula NaI. Sodium hydroxide is a highly corrosive substance. For example 1 mole of Sodium hydroxide is equal to 4000 grams of Sodium hydroxide NaOH molecular weight 4000.
Concentration percentage density Baumé Bé NIST Standard the density of lye and baumé scale degrees. 399971 gmol Appearance White hard when pure opaque crystals Odor. Physical Properties of Sodium Phosphate Na 3 PO 4.
I reach pH around 1365 and not 14. 323 C 613 F. 1388 C 2530 F.
Uses of Acids and Bases. Calculate the average molar concentration of the sodium hydroxide solution. Its usually applied as.
It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. On a commercial scale Caustic soda sodium hydroxide can be prepared by an aqueous solution of sodium. When dissolved in water or neutralized with acid it releases a significant amount of heat which could ignite combustible objects.
149894 Appearance white solid deliquescent. 596 K Boiling point. Sodium Phosphate Structure Na 3 PO 4.
Download Product Safety Card. To calculate the molarity one must first calculate how much Sodium hydroxide is present in 1 L of 50 Sodium hydroxide solution. In simple words 1 mole is equal to the atomic weight of the substance.
Sodium hydroxide solution density table caustic soda lye baumé scale degrees Bé conversion chart. Sodium hydroxide CAS 1310-73-2 pellets for analysis EMSURE - Find MSDS or SDS a COA data sheets and more information. THIS IS THE VALUE THAT YOU WILL USE IN EXPERIMENT 12B.
1661 K Solubility in water. I tried the method which included Molar Massconcentration of KOH 5611085 which leads to 66g. Molecular Weight Molar Mass.
40 gmol Chemical Formula.

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube

Quiz Worksheet Molar Mass Study Com

Sodium Hydroxide Caustic Soda Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs
No comments for "Sodium Hydroxide Molar Mass"
Post a Comment